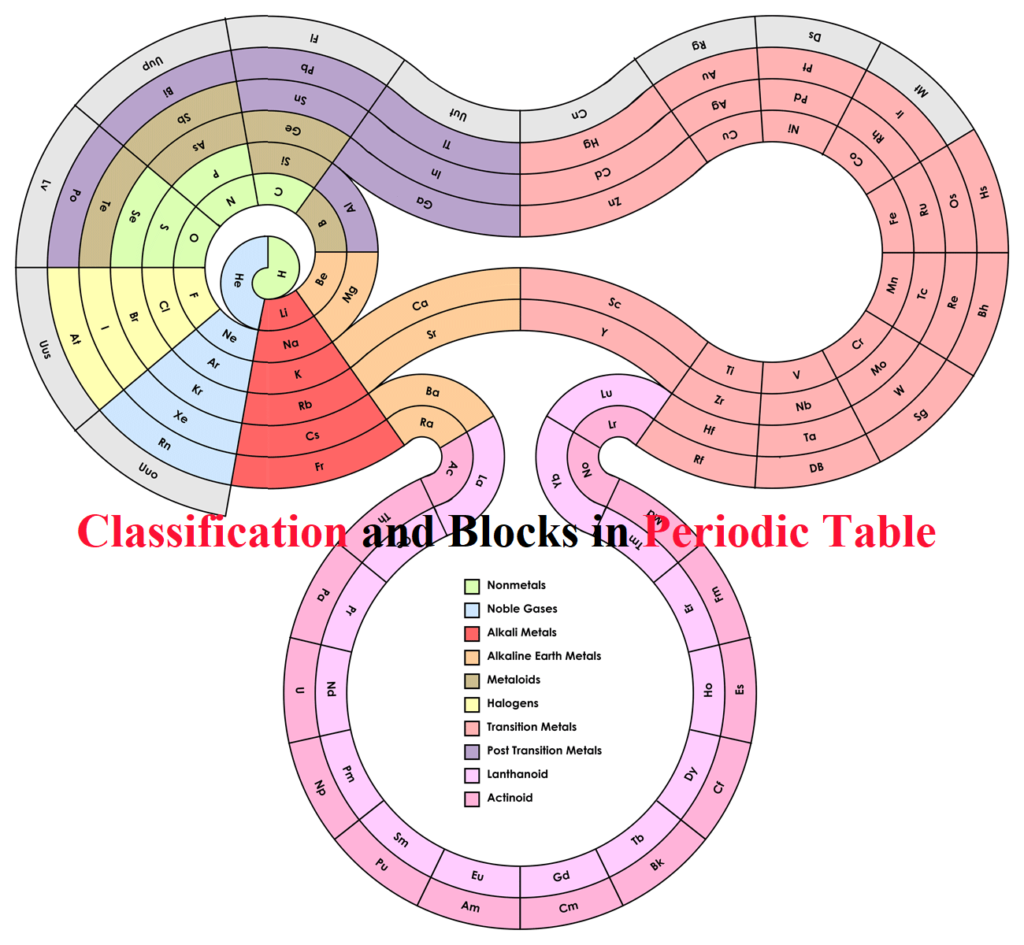
Classification and Blocks in Periodic Table Classification and Blocks in Periodic Table: Topics covered: Earlier classifications Dobereiner’s Triads Newland’s law of octaves Mendeleev’s Periodic Classification Modern Periodic Table or Moseley’s Periodic Law With the discovery of a large number of elements, it became difficult to study the elements individually, so the classification of elements was done to make the study easier. Earlier Classification: Dobereiner’s Triads: In triads, the atomic mass of the middle element is approximately the average of the other two elements. This is known as the Law of Triads. This classification was applicable to very few elements and…
Author: Dr. Vikas Jasrotia
Electrochemical Cells Nernst Equation and Gibbs Energy Syllabus:- Electrolytic cells & Galvanic cells The function of Salt Bridge Redox reaction, EMF of the cell, standard electrode potential Standard hydrogen electrode (SHE) Nernst equation and its application to Chemical cell Equilibrium Constant from Nernst Equation Electrochemical Cell and Gibbs Energy Electrochemistry: It is a branch of chemistry that deals with the relationship between chemical energy and electrical energy and their interconversions. Redox Reactions: Oxidation is the process that involves the loss of electrons & reduction is a process in which it involves the gain of electrons. The reactions which involve…
Solved Questions Structure of Atom Que 1. Neutrons can be found in all atomic nuclei except in one case. Which is this atomic nucleus and what does it consists of? Ans 1. Hydrogen atom. It consists of only one proton. Que 2. Calculate wave number of yellow radiations having a wavelength of 5800 A0. Ans 2. Wave number = 1/ wavelength Wavelength = 5800 A0= 5800 x 10-10 m Wave number = 1/5800 x 10-10 m = 1.72 x 106 m-1 Que 3. What are the values of n and l for 2p orbital? Ans 3. n=2 and l= 1…
Quantum Mechanics and Quantum Numbers Quantum Mechanics and Quantum Numbers post provides complete information about quantum numbers with examples and wave function Quantum Mechanics: Quantum mechanics takes into account the dual behavior of matter. An equation, given by Schrodinger, which has a better physical interpretation in terms of wave properties is Ĥ ψ = Eψ. Where Ĥ is called Hamiltonian operator, E is the total energy of the system (K.E + P.E) and ψ is called the wave function. Physical Significance of ψ: The wave function (ψ) is a mathematical function and it has no physical significance. Wave functions of…
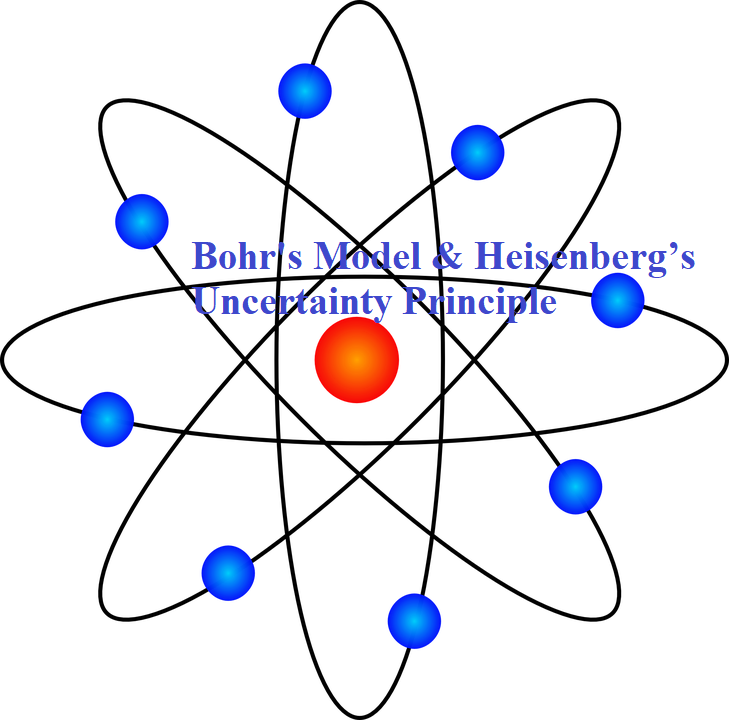
Bohr’s Model & Heisenberg’s Uncertainty Principle Bohr’s Model & Heisenberg’s Uncertainty Principle Bohr’s Model of Atom: The general features of the structure of the hydrogen atom and its spectrum was first explained by Niels Bohr. The important postulates of his theory are: 1. The electron in the hydrogen atom can move around the nucleus in circular paths of fixed radius and energy. These paths are called orbits or stationary states or allowed energy states. These energy levels are numbered as 1,2,3 etc or as K, L, M, N, etc. These numbers are known as Principal quantum numbers. …
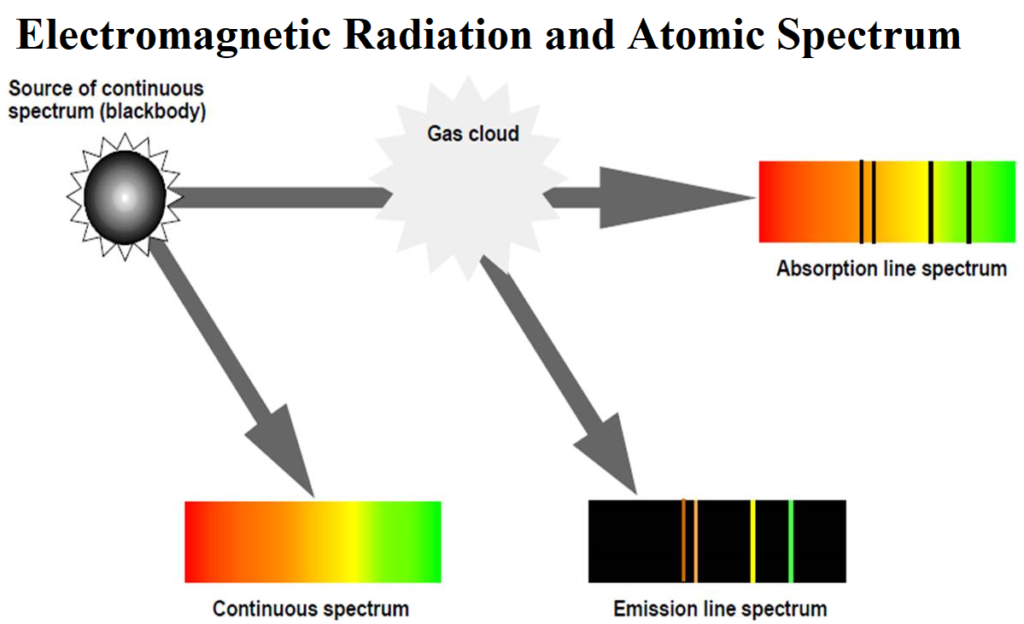
Electromagnetic Radiation and Atomic Spectrum Electromagnetic Radiation and Atomic Spectrum Electromagnetic radiations: The radiations which are associated with electrical and magnetic fields are called electromagnetic radiations. When an electrically charged particle moves under acceleration, alternating electrical and magnetic fields are produced and transmitted. These fields are transmitted in the form of waves. These waves are called electromagnetic waves or electromagnetic radiations. Properties of electromagnetic radiations: (i) Oscillating electric and magnetic field is produced by oscillating charged particles. These fields are perpendicular to each other and both are perpendicular to the direction of propagation of the wave. (ii) They do…
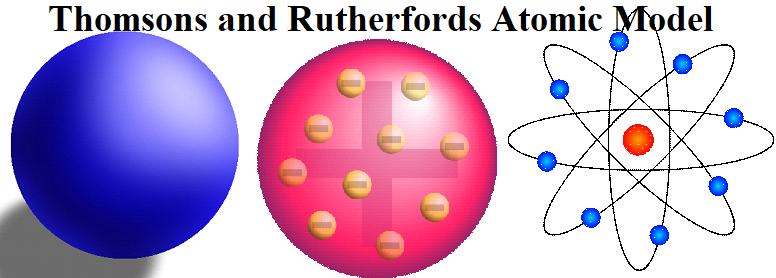
Thomsons and Rutherfords Atomic Model Thomsons and Rutherfords Atomic Model is about the most important atomic models proposed during the study of the structure of atom. Types of Atomic Species: Isotopes: Atoms of the same element having the same atomic number but a different mass number. For example, isotopes of Hydrogen are 11H, 12H, 13H. Isobars: Atoms of different elements having the same mass number but a different atomic number, e.g., 1840Ar, 1940K, 20Ca40 Isotones: Atoms of different elements which contain the same number of neutrons. e.g., 614C, 715N, 816 Isoelectronic Species: Atoms or ions containing the same number…
IS MATTER AROUND US PURE The post IS MATTER AROUND US PURE contains questions about the chapter. Smoke and fog both are aerosols. In what way are they different? Why do we call sugar a pure substance? What are saturated and unsaturated solutions? Define a solution What is a suspension? Give its example and Define the concentration of a What is the Tyndall effect? What is the difference between True solutions and colloids? What are alloys? Why are alloys called a mixture? Write the characteristics of Define solute and What is solubility? Give properties of a true solution. Explain why particles…
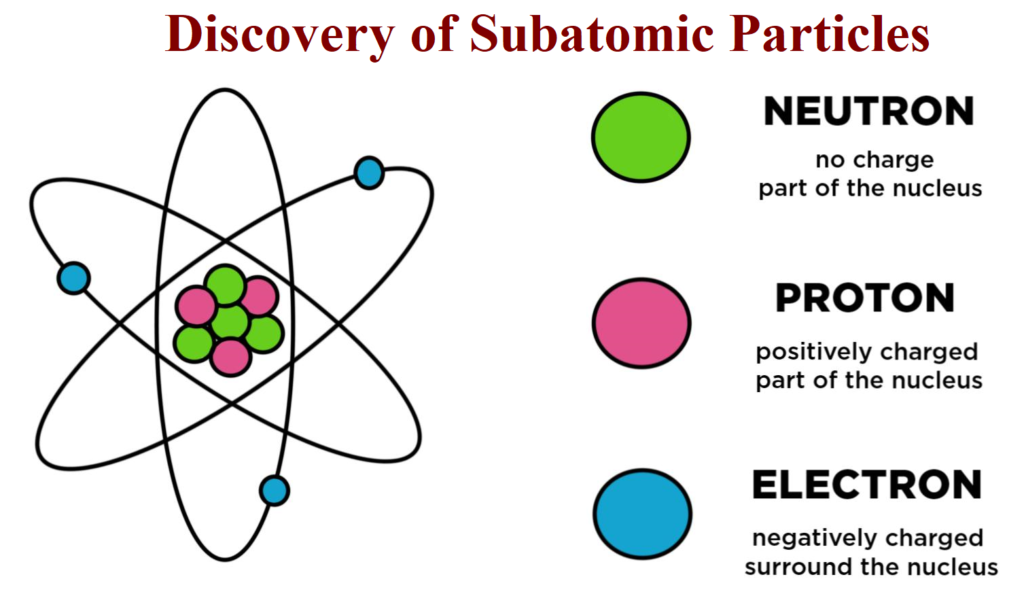
The Discovery of Subatomic Particles This post The Discovery of Subatomic Particles and Atomic Models is all about the subatomic particles their discoveries along with their charges. STRUCTURE OF ATOM Structure of Atom The term atom was introduced by Dalton and is defined as the smallest particle of an element that retains all its properties and identity during a chemical reaction. SUB – ATOMIC PARTICLE Discovery of Electron Electron was discovered by J J Thomson by Cathode ray discharge tube experiment. A cathode-ray tube is made of glass containing two thin pieces of metal (called electrodes) sealed in…
Solved MCQs States of Matter All questions of Solved MCQs States of Matter are asked in various competitive examinations. Q1. A sample of gas occupies 100 mL at 27 0C and 740 mm pressure. When its volume is changed to 80 mL at 740 mm pressure, the temperature of the gas will be (a) 21.6 0C (b) 240 0C (c) – 33 0C (d) 89.5 0C Ans 1. (c) – 33 0C Since, Pressure is same According to Charles law V1/T1 = V2/T2 (T1 = 27 + 273 = 300 K) 100/300 = 80/T2 T2 = 240 K…