CBSE Important Questions Electrochemistry CBSE Important Questions Electrochemistry Que 1. Why conductivity of a solution does decrease with dilution? Ans…
Browsing: Electrochemistry
Electrolysis of an Aqueous Solution of CuSO4 Que. What will happen during the electrolysis of an aqueous solution of CuSO4 in…
Electrolysis and Faraday’s Laws of Electrolysis Electrolysis and Faraday’s Laws The process of decomposition of an electrolyte on passing an…
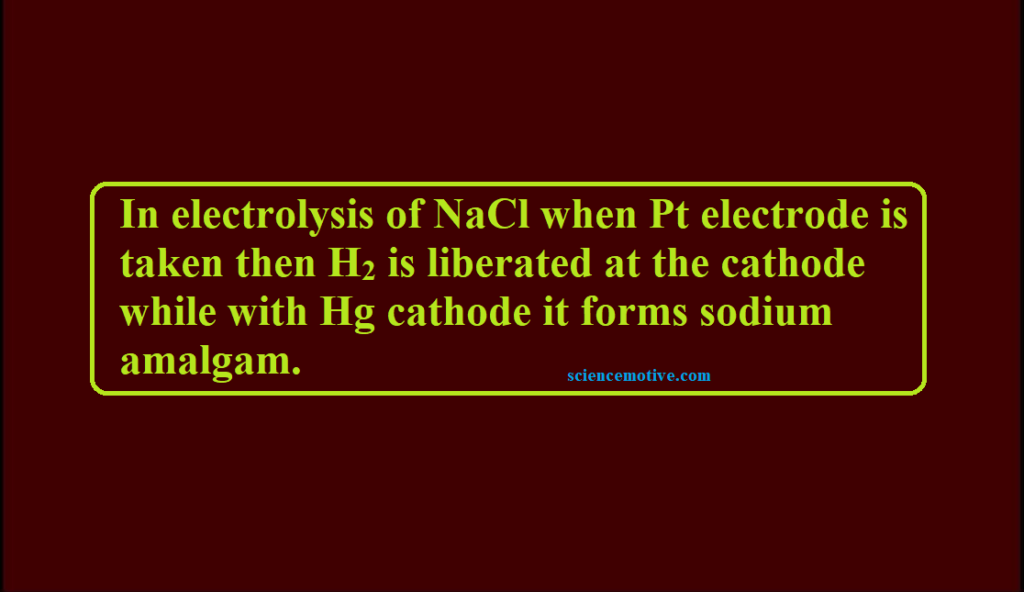
Electrolysis of NaCl When Pt Electrode Que. In electrolysis of NaCl when Pt electrode is taken then H2 is liberated…
MCQ on Electrochemistry Class 12 with Answers MCQ on Electrochemistry Class 12 with Answers Que 1. When the sample of…
Electrochemistry Class 12 NCERT Exercise Solutions Que 1. Arrange the following metals in the order in which they displace each…
NCERT Intext Questions Class 12 Electrochemistry Que 1. How would you determine the standard electrode potential of the system Mg2+|Mg?…
Important Assertion Reason Questions of Electrochemistry Que 1. Assertion: In an electrochemical cell anode and cathode are respectively negative and…
Electrochemistry Class 12 Notes Syllabus:- Electrolytic cells & Galvanic cells The function of Salt Bridge Redox reaction, EMF of…
Electrochemistry Class XII Solved Numericals Que 1. 0.05 M NaOH solutions offered resistance of 31.16 Ω in a conductivity cell…