Solved Subjective and Objective Questions of Amines Solved Subjective and Objective Questions of Amines: Q1. Why Amines are basic in nature? Ans 1. The reaction of amines with mineral acids to form ammonium salts shows that these are basic in nature. Amines have an unshared pair of electrons on nitrogen atom due to which they behave as Lewis base. Q2. In Alkylamines and ammonia which is more basic and why? Ans 2. Due to the electron releasing nature of the alkyl group, it (R) pushes electrons towards nitrogen and thus makes the unshared electron pair more available for sharing with…
Author: Dr. Vikas Jasrotia
Intermolecular Forces and The Gas Laws Intermolecular Forces and The Gas Laws Introduction: Matter is anything that occupies space and has a definite mass. Matter mainly exists in three different states – solid, liquid, and gaseous states. Solids have a definite shape and definite volume. This is because in solids the particles are closely packed and so the intermolecular force of attraction is greater. Liquids have no definite shape but have a definite volume. In liquids, the intermolecular force of attraction is smaller than that in solids. So, the particles do not have a fixed position. Gases have no definite…
Solved Important Questions d & f Block Elements Solved Important Questions d & f Block Elements: Que 1. Cu+ is not stable in an aqueous solution. Why? Ans 1. In an aqueous solution, Cu+ undergoes disproportionation to form a more stable Cu2+ ion. 2Cu+ (aq) → Cu2+ (aq) + Cu(s) The higher stability of Cu2+ in an aqueous solution may be attributed to its greater negative ∆hydH than that of Cu+. It compensates for the second ionization enthalpy of Cu involved in the formation of Cu2+ ions. Que 2. Which is a stronger reducing agent Cr2+ or Fe2+ and why?…
Solved MCQs d & f Block Elements Solved MCQs d & f Block Elements: Que 1. Four successive members of the first-row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionisation enthalpy? a) Vanadium (Z = 23) b) Chromium (Z = 24) c) Manganese (Z = 25) d) Iron (Z = 26) Ans 1. (c) It can be seen after losing 2 electrons, Mn has 3d5 configuration which is exactly half filled. More energy is required to remove the third electron. Que 2. The aqueous solution containing…
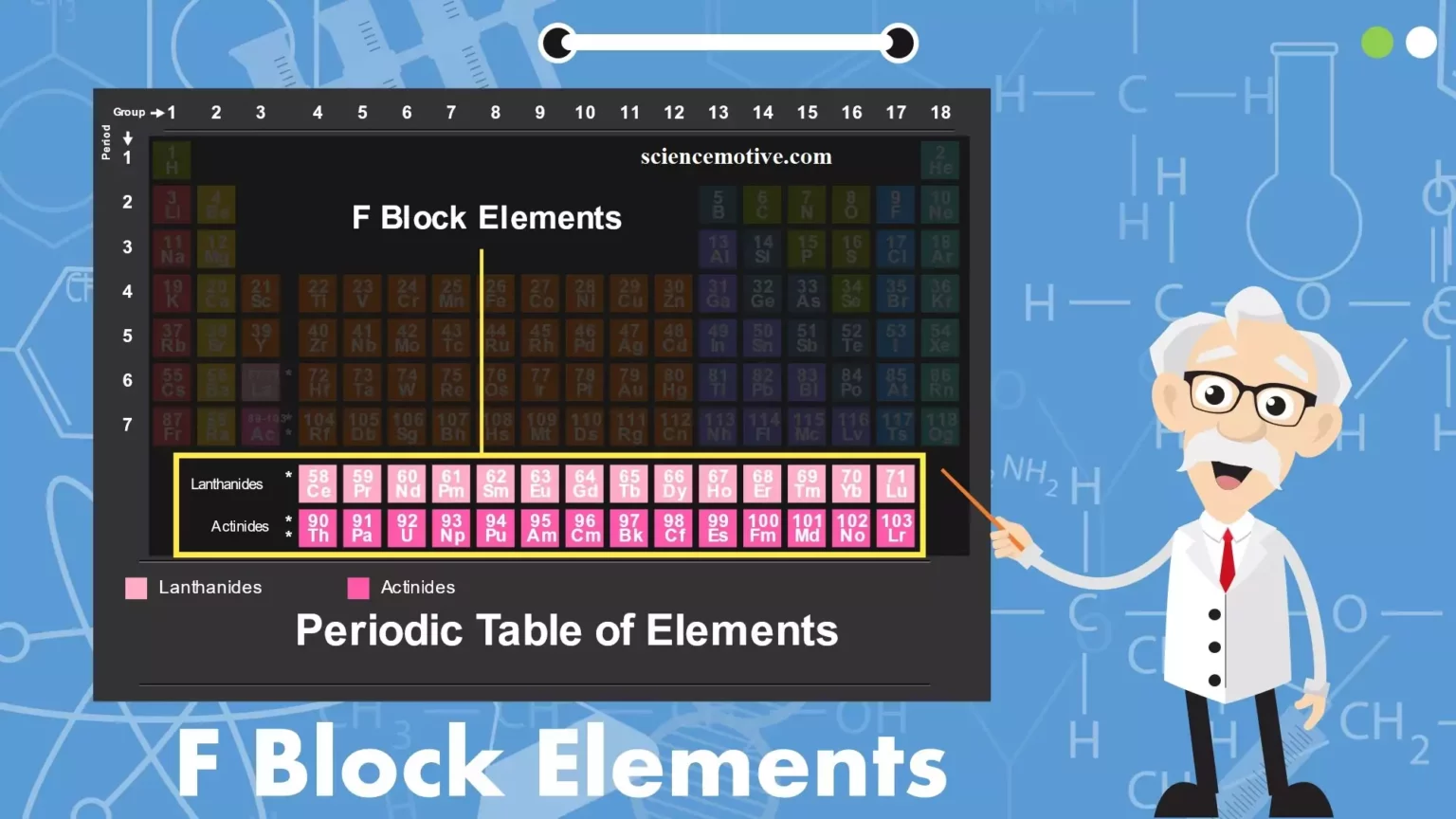
f-Block Elements and Properties f-Block Elements and Properties: The elements in which the last electron enters in the anti-penultimate f-subshell are called f-block elements. They include lanthanides of the 6th period and actinides of the 7th period. The Lanthanoids: Elements’ filling up of 4f – orbitals are called Lanthanoids series. The 14 elements after lanthanum of the 6th period are called lanthanides or lanthanoids or lanthanones or rare earths. They include elements from 58Ce to 71Lu. They are generally represented as Ln. (i) Atomic and Ionic Radii: The overall decrease in atomic and ionic radii from Lanthanum to Lutetium is…
Preparation and Properties of Potassium Permanganate and Potassium Dichromate Preparation and Properties of Potassium Permanganate and Potassium Dichromate: General Properties of First Row Transition Metal Compounds Oxides and oxometal ions: (i) Oxides of metals in low oxidation states + 2 and + 3 (MO, M3O4 and M2O3) are generally basic except Cr2O3 which is amphoteric in character. (ii) Oxides of metals in higher oxidation states + 5 (M2O5, MO3, M2O7) are generally acidic in character. (iii) Oxides of metals in their intermediate oxidation states + 4 (MO2) are generally amphoteric in nature. Besides the oxides, the oxocations, which stabilise V…
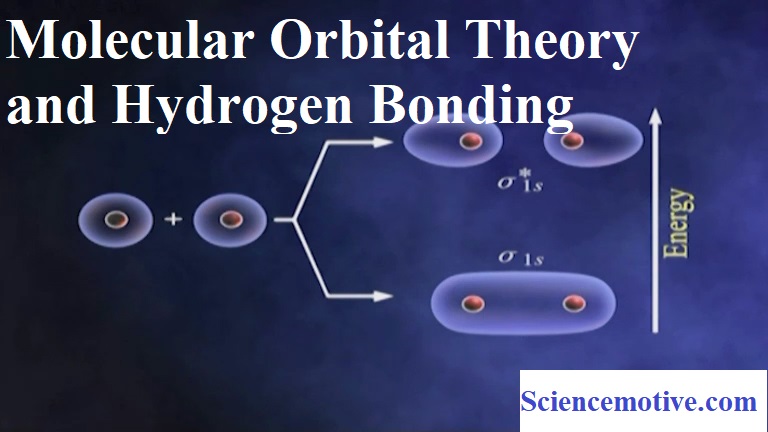
Molecular Orbital Theory and Hydrogen Bonding Molecular Orbital Theory and Hydrogen Bonding: Molecular Orbital Theory: This theory was developed by F.Hund and R.S Mulliken. The important postulates of this theory are: (i) When two atomic orbitals combine or overlap, they lose their identity and form new orbitals. The new orbitals formed are called molecular orbitals. (ii) Only those atomic orbitals can combine to form molecular orbitals which have comparable energies and proper orientations. (iii) The number of molecular orbitals formed is equal to the number of combining atomic orbitals. (iv) When two atomic orbitals combine, they form two new orbitals…
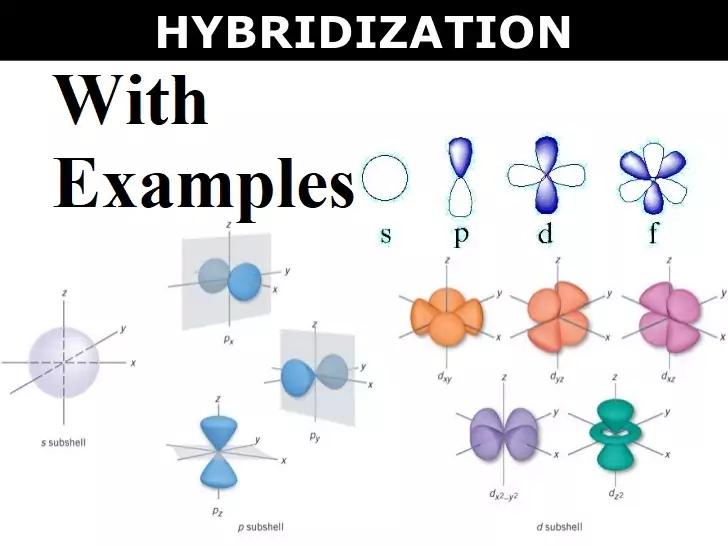
Concept of Hybridisation with Examples Concept of Hybridisation with Examples: Hybridization: It can be defined as the process of intermixing of the orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of a new set of orbitals of equivalent energies and shape. Salient Features of hybridization: (i) The number of hybrid orbitals is equal to the number of atomic orbitals that get hybridized. (ii) The hybridized orbitals are always equivalent in energy and shape. (iii) The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals. (iv) These hybrid orbitals…
Transition elements and General Characteristics Transition elements and General Characteristics: Introduction The elements lying in the middle of periodic table belonging to groups 3 to 12 are known as d – block elements. The elements in which the last electron enters into the d-orbitals of penultimate shell i.e. (n–1) d where n is the last shell are called d-block elements. They are placed in between s-block and p-block elements. They show a regular transition from the highly electropositive metals of s-block elements to the less electropositive p-block elements. So, they are called transition elements. Transition elements A transition element is…
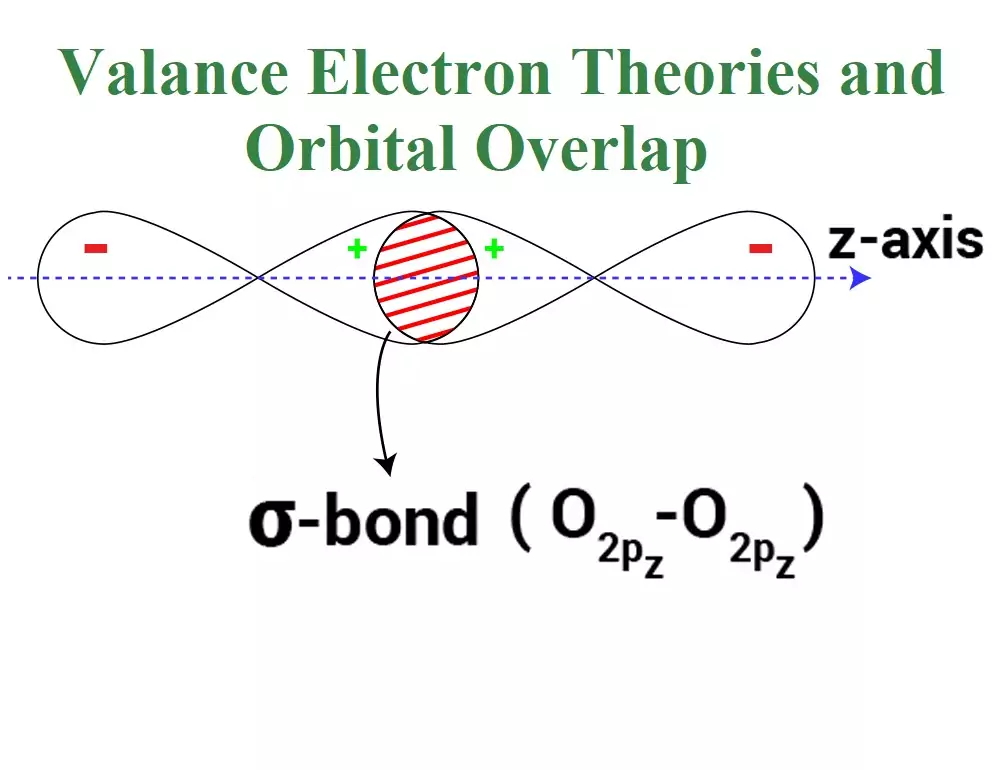
Valance Electron Theories and Orbital Overlap Valance Electron Theories and Orbital Overlap: The valence shell electron pair repulsion (VSEPR) Theory: This theory was proposed by Sidgwick and Powell and later modified by Nyholm and Gillespie. The important postulates of this theory are: (i) The shape of a molecule depends upon the number of valence shell electron pairs (bonded or nonbonded) around the central atom. (ii) Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged. (iii) These pairs of electrons tend to occupy such positions in space that minimize repulsion and thus maximize…