f-Block Elements and Properties
f-Block Elements and Properties:
The elements in which the last electron enters in the anti-penultimate f-subshell are called f-block elements. They include lanthanides of the 6th period and actinides of the 7th period.

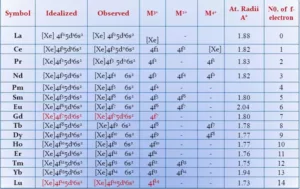
The Lanthanoids: Elements’ filling up of 4f – orbitals are called Lanthanoids series. The 14 elements after lanthanum of the 6th period are called lanthanides or lanthanoids or lanthanones or rare earths. They include elements from 58Ce to 71Lu. They are generally represented as Ln.
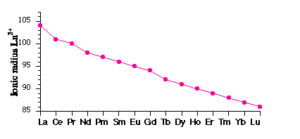
(i) Atomic and Ionic Radii: The overall decrease in atomic and ionic radii from Lanthanum to Lutetium is a unique feature in the chemistry of the lanthanoids. This regular decrease is known as lanthanoid contraction. In lanthanides, as the atomic number increases, the nuclear charge increases one by one and the electrons are added to the anti-penultimate f subshell. Due to its diffused shape, f orbitals have poor shielding effect. So the nucleus can attract the outermost electrons strongly and as a result, the radii decreases.
Consequences of Lanthanoid Contraction:
a) It results in a slight variation in their chemical properties which helps in their separation by ion-exchange methods.
b) Each element beyond lanthanum has same atomic radius as that of the element lying above it in the same group (e.g., Zr 145 pm, Hf 144 pm); Nb 134 pm, Ta 134 pm; Mo 129 pm, W 130 pm).
c) The covalent character of hydroxides of lanthanoids increases as the size decreases from La3+ to Lu3+. Hence, the basic strength decreases. Thus, La(OH)3 is most basic whereas Lu(OH)3 is least basic. Similarly, the basicity of oxides also decreases in the order from La3+ to Lu3+.
d) Tendency to form stable complexes from La3+ to Lu3+ increases as the size decreases in that order.
(ii) Oxidation State: In lanthanoids, the most common oxidation state is +3. However, +2 and +4 ions in solution or in solid compounds are also obtained. This irregularity arises mainly from the extra stability of empty, half-filled or filled f subshells. Cerium shows the oxidation state +4 due to its noble gas configuration. Pr, Nd, Tb and Dy also exhibit +4 state but only in oxides, MO2. Eu and Yb show +2 oxidation state because of the stable f7 or f14 configuration. Sm shows +2 oxidation state also.
(iii) Colour: Most of the trivalent lanthanoid ions are coloured both in the solid-state and in an aqueous solution. This is due to the partly filled f-orbitals which permit f-transition.
La3+ (colourless) Lu3+ (colourless)
Ce3+ (colourless) Yb3+ (colourless)
Pr3+ (yellow green) Tm3+ (green)
Nd3+ (red) Er3+ (pink)
Pm3+ (uncertain) Ho3+ (Yellow)
Sm3+ (yellow) Dy3+ (yellow)
Eu3+ (pink) Tb3+ (pink)
Gd3+ (pink)
(iv) Complex Formation: Although the lanthanoid ions have a high charge (+3) yet the size of their ions is very large yielding small charge to size ratio, i.e., low charge density. As a consequence, they have a poor tendency to form complexes.
(v) Reducing Character: The E° values for M3+; M3+(aq) + 3e− → M(s) lie in the range of – 2.2 to -2.4 V (the exception being Eu, E° = – 2.0V) indicating thereby that they are highly electropositive, readily lose electrons and thus are good reducing agents.
Chemical Behaviour:
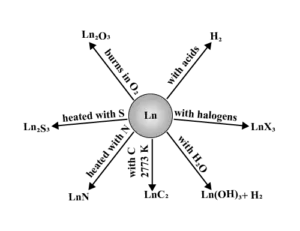

The Actinoids: The elements with atomic numbers 90 to 103, i.e., thorium to lawrencium (which come immediately after actinium, Z = 89 are called actinoids or actinides or actinones. These elements involve the filling of 5f-orbitals and are also called 5f-block elements or second inner transition series. Their general electronic configuration is [Rn] 5f1−14 6d0−1 7s2.
Most of them are artificially prepared and are short-lived. They are radioactive. The elements after Uranium are artificially prepared and so they are called trans-uranium elements or trans-uranic elements.
(i) Atomic and Ionic Radii: In actinoid series, the atomic and ionic radii decrease regularly from left to right. This is known as Actinoid contraction.
(ii) Oxidation States: Exhibition of a large number of oxidation states of actinoids is due to the fact that there is a very small energy gap between 5f, 6d and 7s subshells and thus all their electrons can take part in bond formation.
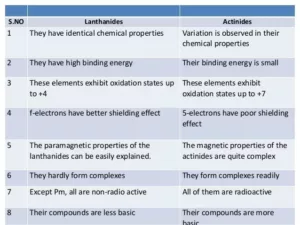
Comparison between Lanthanoids and Actinoids
Uses of Actinoids:

f-Block Elements and Properties