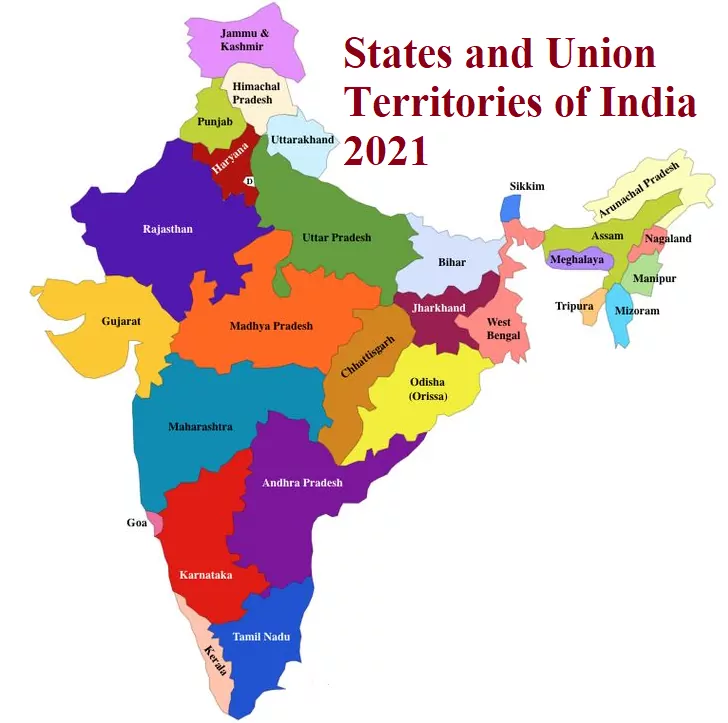
States and Union Territories of India 2021 States and Union Territories of India 2021 S.No States Name Capital Founded on Area (Km2) 1 Andhra Pradesh Hyderabad (Proposed Capital Amaravati) 1 Nov. 1956 160,205 2 Arunachal Pradesh Itanagar 20 Feb. 1987 83,743 3 Assam Dispur 26 Jan. 1950 78,438 4 Bihar Patna 26 Jan. 1950 94,163 5 Chhattisgarh Raipur 1 Nov. 2000 135,191 6 Goa Panaji 30 May. 1987 3,702 7 Gujarat Gandhinagar 1 May. 1960 196,024 8 Haryana Chandigarh 1 Nov. 1966 44,212 9 Himachal Pradesh Shimla 25 Jan. 1971 55,673 10 Jharkhand Ranchi 15 Nov. 2000 79714 11 Karnataka…
Author: Dr. Vikas Jasrotia
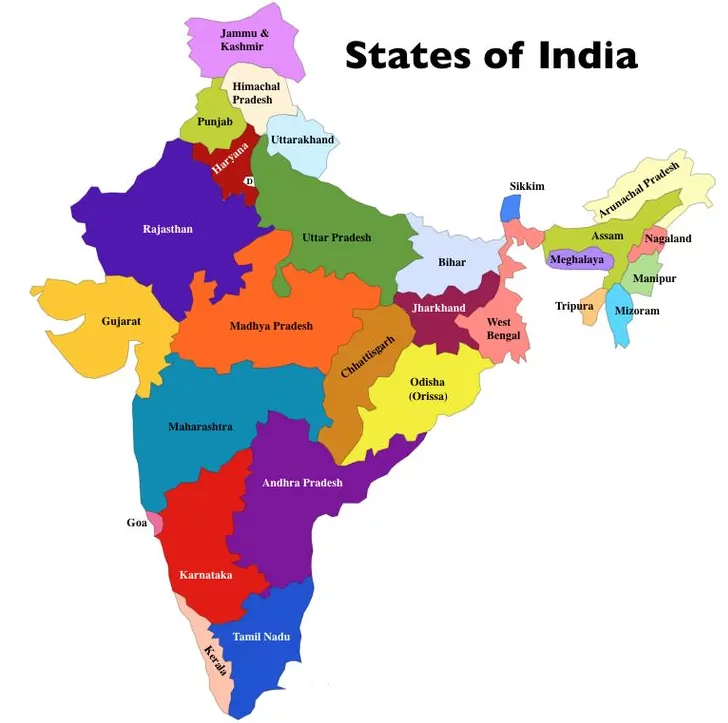
States and Capitals of India 2021 States and Capitals of India 2021 S.No States Name Capital Founded on Area (Km2) 1 Andhra Pradesh Hyderabad (Proposed Capital Amaravati) 1 Nov. 1956 160,205 2 Arunachal Pradesh Itanagar 20 Feb. 1987 83,743 3 Assam Dispur 26 Jan. 1950 78,438 4 Bihar Patna 26 Jan. 1950 94,163 5 Chhattisgarh Raipur 1 Nov. 2000 135,191 6 Goa Panaji 30 May. 1987 3,702 7 Gujarat Gandhinagar 1 May. 1960 196,024 8 Haryana Chandigarh 1 Nov. 1966 44,212 9 Himachal Pradesh Shimla 25 Jan. 1971 55,673 10 Jharkhand Ranchi 15 Nov. 2000 79714 11 Karnataka Bengaluru(Formerly Bangalore)…
Electrochemistry Class XII Solved Numericals Que 1. 0.05 M NaOH solutions offered resistance of 31.16 Ω in a conductivity cell at 298 K. If cell constant is 0.367 cm–1. Find out the molar conductivity of NaOH solution. Ans 1. Given Molarity = 0.05 M Cell Constant = 0.367 cm-1 Que 2. Consider the following cell reaction: 2Fe(s) + O2 (g) + 4H+ (aq) → 2Fe2+ (aq) + 2H2O(l); E0 = 1.67V. At [Fe2+] = 10–3 M, p(O2) = 0.1 atm and pH = 3. What will be the cell potential at 250C? Ans 2. Here n = 4, and [H+]…
Electrochemistry Important questions for Board Que 1. i) Define the molar conductivity of a solution and explain how molar conductivity changes with a change in concentration of solution for a weak and strong electrolyte. ii) The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500Ω. What is the cell constant, if the conductivity of 0.01 M KCl solution at 298 K is 0.146 × 10–3 S cm–1? Ans 1. i) Molar conductivity – it is defined as the conductance of the solution which contains one mole of an electrolyte such that the entire solution…
Electrochemistry Neet Important Solved Questions Que 1. Which of the following statements is incorrect regarding electrochemistry? (a) It is the study of the production of electricity from the energy released during spontaneous chemical reactions. (b) NaOH, Cl2, alkali, and alkaline earth metals are prepared by electrochemical methods. (c) The demerit associated with electrochemical methods is that they are more polluting. Thus they are eco[1]destructive. (d) Electrochemical reactions are more energy-efficient and less polluting. Ans 1. (c) Electrochemical reactions are more energy-efficient and less polluting. Thus the study of electrochemistry is important to create new eco-friendly technologies. Que 2. For a…
Solutions Chemistry Important NEET Questions Que 1. How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3? The concentrated acid is 70% HNO3. (a) 70.0 g conc. HNO3 (b) 54.0 g conc. HNO3 (c) 45.0 g conc. HNO3 (d) 90.0 g conc. HNO3 Ans 1. (c) Explanation: Que 2. In water-saturated air, the mole fraction of water vapour is 0.02. If the total pressure of the saturated air is 1.2 atm, the partial pressure of dry air is (a) 1.18 atm (b) 1.76 atm (c) 1.176 atm (d) 0.98 atm.…
Solutions Chemistry Class 12 Important Questions with Answers Q1. a) Calculate the molarity of a sulphuric acid solution in which the mole fraction of water is 0.85. b) The graphical representation of vapour pressure of two-component system as a function of composition is given alongside. i) Are the A – B interactions weaker, stronger or of the same magnitude as A–A and B–B ii) Name the type of deviation shown by this system from Raoult’s law. iii) Predict the sign of ∆mixH for this system. iv) Predict the sign of ∆mixV for this system. v) Give an example of such…
JEE and NEET Important Questions Chapter Solutions Que 1. Which of the following units is useful in relating the concentration of a solution with its vapour pressure? (i) Mole fraction (ii) Parts per million (iii) Mass percentage (iv) Molality Ans 1. (i) Mole fraction is useful in relating vapour pressure with the concentration of the solution. According to Raoult’s law, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction Que 2. On dissolving sugar in water at room temperature solution feels cool to touch. Under which of the following cases…
Ncert Chemistry Class 12 Questions Solutions Chapter 2 NCERT Intext Questions Chapter – Solutions Que 1. Calculate the mass percentage of benzene (C6H6) and carbon tetrachloride (CCI4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride. Ans 1. Mass of solution = Mass of Benzene (C6H6) + Mass of Carbon Tetrachloride (CCl4) = 22 g +122 g = 144 g Mass percentage of Benzene = 22/144 × 100 =15.28 % Mass % of CCl4 = 122/144 × 100 = 84.72 % Que 2. Calculate the mole fraction of benzene in solution containing 30% by mass in…
NCERT Class 12 Chemistry Chapter 2 Exercise Solutions Que 1. Define the term solution. How many types of solutions are formed? Write briefly about each type with an example. Ans 1. A solution is a homogeneous mixture of two or more chemically non-reacting substances. Types of solutions: There are nine types of solutions. Types of Solution Examples Gaseous solutions (a) Gas in gas Air, a mixture of 02 and N2, etc. (b) Liquid in gas Water vapour (c) Solid in gas Camphor vapours in N2 gas, smoke etc. Liquid solutions (a) Gas in liquid C02 dissolved in water (aerated water), and…