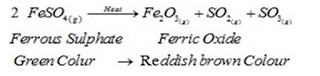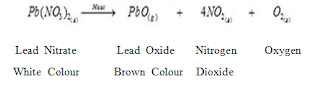Chemical Reactions And Equations Class 10 Notes
Chemical Reactions And Equations Class 10 Notes
Physical Change Change in physical properties.
- Melting
- Boiling
- Condensation
Note- No change occurs in the identity of the substance
Chemical Change
- Atoms in the reactants are rearranged to form one or more different substances.
- Old bonds are broken, new bonds are formed.
- Reactants lose their properties to form products of different properties.
Examples –
- Cooking of food
- Rusting of iron
- Heating of Lead nitrate
- Souring of milk
- Ripening of fruit.
Rusting of iron is a chemical change because
- A new substance iron oxide is formed. The change is permanent; the article has got a rust layer (which may only peal off).
- There is an increase in mass when rust forms.
- An energy change has taken place (which may not be visible).
4 Fe(s) + 3 O2 (g) —> 2 Fe2O3 (rust) Iron Oxygen Ferric oxide
Chemical reaction:
Chemical changes or chemical reactions are the changes in which one or more new substances are formed. e.g. Food gets digested in our body.
- Rusting of iron.
- Magnesium is burnt in air to form magnesium oxide. This chemical reaction can be represented as 2 Mg + O2 –> 2MgO
- We can observe or recognize a chemical reaction by observing changes in state, colour, the evolution of gas, or changes in temperature.
- the How do anabolic steroids work in the brain? | National Institute on Drug Abuse tri tren 250 a pharma merger’s impact ripples across west virginia as viatris plant closes | biopharma dive physical state of the reactants and the products are mentioned to make the chemical reactions more informative. e.g. we use (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous.

Chemical Equations Representation of a chemical reaction in terms of symbols and formula of the reactants and products is known as a chemical equation.
Features of a chemical equation:
- The reactants are written on the left-hand side with a plus sign between them.
- The products are written on the right-hand side with a plus sign between them.
- An arrow separates the reactants from the products. The arrowhead points towards the products and indicates the direction of the reaction.
Skeletal chemical equation
- A chemical equation that simply represents the symbols and formulas of reactants and products taking part in the reaction is known as the skeletal chemical equation for a reaction.
- For example: For the burning of Magnesium in the air, Mg + O2 à MgO is the skeletal equation.
Balanced Equation
A balanced equation is one in which the number of atoms on the reactant and product sides is equal.
Or
The number of atoms of an element on the reactant side = number of atoms of that element on the product side.
- The simple form of representation of a chemical reaction in words is known as a word equation.
- Magnesium + Oxygen ———–> Magnesium Oxide
- The representation of a chemical reaction with the help of chemical formula is called a chemical equation.
- 2Mg + O2→ 2MgO
Writing Chemical Equations
- In a chemical reaction, the reactants are written on the left-hand side and the products on the right-hand side of the equation.
- An arrow (→) pointing towards the products is inserted between the reactants and the products. It also represents the direction of the reaction.
- A single arrow (→) indicates the direction in which the reaction proceeds.
- A double arrow( ↔ ) indicates a reversible reaction, i.e. the products recombine to form the reactants.
- A plus sign (+) is inserted between two or more reactants or products formed.
- If reactions are carried out under specific conditions of temperature, pressure, catalyst etc., then these conditions are mentioned on the arrow.
- The chemical equation can be made more informative by mentioning the physical states of the reactants and products.
- If gas is liberated as a product then it is represented by an arrow pointing upwards (↑). If the product formed is in the form of a precipitate, it is represented by an arrow pointing downwards (↓).
Steps Involved in Chemistry Balancing Chemical Equations Practice
Consider the chemical reaction between magnesium and oxygen to understand the steps involved in balancing a chemical equation. Step 1 Let us first write the word equation for this reaction. Magnesium + Oxygen→Magnesium oxide
Step 2 Write the chemical equation for the reaction between magnesium and oxygen. Mg + O2 → MgO
Step 3 Count the number atoms of an element occurring on both L.H.S. and R.H.S. in this equation. Mg + O2 → MgO
| Component | Reactant | Product |
| Magnesium | 1 | 1 |
| Oxygen | 2 | 1 |
This is an unbalanced equation.
Step 4
To balance a chemical equation, first draw boxes around each formula. Do not change anything inside the boxes while balancing the equation.

- Choose a reactant or a product that has the maximum number of atoms in it. In that compound, select the element which has the maximum number of atoms. In this equation, we shall select MgO i.e. magnesium oxide, and the element oxygen in it.
- To balance the oxygen atoms, let us multiply the magnesium oxide molecule by 2 on the right-hand side. The equation can now be expressed as,

| Component | Reactant | Product |
| Magnesium | 1 | 1 x2 = 2 |
| Oxygen | 2 | 1 x 2 = 2 |
Step 5
There are two oxygen atoms on either side of the equation but one magnesium atom on the reactant’s side and two on the product’s side. Therefore, multiply the magnesium atom by 2 on the left-hand side.
| Component | Reactant | Product |
| Magnesium | 1 x 2 = 2 | 2 |
| Oxygen | 2 | 2 |
A balanced equation is,

The number of atoms of each element of reactants = The number of atoms of each element of products
Step 6
Writing Specific Conditions on the Arrow

The reaction is carried out in the presence of ‘Heat’. On heating, magnesium combines with oxygen present in air to form magnesium oxide.
Step 7
Writing Symbols of Physical States

Using these steps, you can balance any chemical equation.
Chemistry Balancing Chemical Equations Practice :
Que1. Why do we need to balance a chemical equation?
Que2. Balance the following equations:
i) Fe + H2O —–> Fe2O3 + H2
ii) Zn + HNO3 ——> Zn(NO3)2 + H2O + N2O
iii) NaHCO3 ——> Na2CO3 + H2O + CO2
Chemical Reactions And Equations Class 10 Notes
Types of Chemical Reactions
i). Combination Reaction
ii). Decomposition Reaction
a). Thermal Decomposition
b). Electrolytic Decomposition
iii). Displacement Reaction OR Substitution reaction
iv). Double Displacement Reaction
a). Precipitation Reaction
b). Neutralisation Reaction
v). Redox Reaction
vi). Oxidation Reaction
vii). Reduction Reaction
viii). Endothermic Reaction
ix). Exothermic Reaction
Explanation of the types of Chemical reactions i.e Combination, Decomposition, Displacement And Double Displacement reactions using examples:
i). Combination Reaction:
The reactions in which two or more substances combine to form a new substance are called combination reactions. For example: a). 2 Mg (s) + O2 (g) → 2 MgO
Magnesium Oxygen Magnesium oxide (White ash)
(basic) turns red litmus blue
b). In the laboratory, iron sulphide is prepared by mixing iron and sulphur.
Fe(s) + S(s) → FeS(s)
c). Burning of carbon monoxide in oxygen to form carbon dioxide.
2CO (g) + O2(g) → 2CO2 (g)
d). Calcium oxide upon reaction with water produces calcium hydroxide.
CaO + H2O → Ca(OH)2
Calcium Oxide Water Calcium Hydroxide
(Quick Lime) (Slaked Lime)
Chemical Reactions And Equations Class 10 Notes
ii). Decomposition reactions:
These are opposite to combination reactions. A single compound decomposes or breaks down to give two or more simpler substances. It is of the following types:
I). Thermal Decomposition: When a decomposition reaction is carried out by heating. For Example:
- Mercuric oxide, when heated, undergoes thermal decomposition, to give mercury and oxygen

iii). Displacement Reaction OR Substitution reaction:
A more reactive element (metal) displaces less reactive element (metal) from its aqueous salt soln. For example:
- Zn (s) + FeSO4 (aq) → ZnSO4 (aq) + Fe (s)
(green) (Colourless)
- Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s) ↓
Iron Blue Green Reddish Brown
- Mg (s) + H2SO4 (aq) → MgSO4 (aq) + H2O (g)
Magnesium Sulphuric Magnesium Water
Acid Sulphate
- Cu (s) + 2AgNO3 (aq) → Cu (NO3)2 (aq) + 2Ag (s).
Copper Silver Nitrate Copper Nitrate Silver
- KI (aq) + Cl2 (g) → KCl (aq) + I2 (g)
Potassium Chlorine Potassium Iodine
Iodide Chloride
iv). Double Displacement Reaction:
The chemical reactions in which compounds react to form two different compounds by mutual exchange of ions are called double displacement reactions.
Reactions occur by two different ways
a). Precipitation: In such reactions due to the exchange of ions some insoluble material is formed. This insoluble material is called precipitate and the reaction is called a precipitation reaction. For example:
- ZnSO4 (aq) + BaCl2 (aq) → ZnCl2 (aq) + BaSO4 (s)
Zinc Barium Zinc Barium
Sulphate Chloride Chloride Sulphate (White)
- 2 HCl (aq) + Pb(NO3)2(aq) → 2 HNO3 (aq) + PbCl2 (s) ↓
Hydrochloric Lead Nitric Lead (White)
Acid Nitrate Acid Chloride
- Pb(NO3)2(aq) + 2 KI (aq) → PbI2 (aq) ↓ + KNO3 (s)
Lead Potassium lead Potassium
Nitrate Iodide Iodide Nitrate
b) Neutralization Reaction: In this type of reaction an acid reacts with a base to form salt and water by exchange of ions.
- NaOH (aq) + HCl (aq) → NaCl (aq) + H2O
Sodium Hydrochloric Sodium Water
Hydroxide Acid Chloride
(Base) (Acid) (Salt) (Water)
- ZnO + HNO3 → Zn(NO3)2 + H2O
Zinc Nitric Zinc Water
Oxide Acid Nitrate
Chemical Reactions And Equations Class 10 Notes
Redox Reaction, Oxidation and Reduction Reactions
This part of the types of chemical reaction of chapter Chemical Reactions and Equations involve the types Redox Reaction, Oxidation and Reduction Reactions with exact equations.
v). Redox Reaction: A reaction in which reduction and oxidation takes place simultaneously.
- ZnO (s) + C (s) → Zn (s) + CO (g)
Zinc Carbon Zinc Carbon
Oxide Monoxide
vi). Oxidation Reaction: Oxidation is the gain of oxygen. For example:
- 2 Cu (s) + O2 (g) → 2 CuO (s) (Black)
Copper Oxygen Copper Oxide
- 2 Mg(s) + O2 (g) → 2MgO (s)
Magnesium Oxygen Magnesium Oxide
OR
Removal of hydrogen is also oxidation. For Example:
- 2HI (g) → H2 (g) + I2 (g)
Hydrogen Hydrogen Iodine
Iodic Acid
VII). Reduction Reaction: Reduction is the gain of hydrogen. For example:
- H2 (g) + Cl2 (g) → 2 HCl
Hydrogen Chlorine Hydrochloric Acid
OR
Removal of oxygen is also reduction. Foe Example:
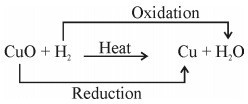
- CuO (s) + CO (g) → Cu (s) + CO2 (g)
Copper Oxide Carbon Copper Carbon Dioxide
(Black) Monoxide (Reddish Brown)
- ZnO (s) + C (s) → Zn (s) + CO (g)
Zinc Carbon Zinc Carbon
Oxide Monoxide
An oxidation-reduction (redox) reaction is a type of chemical reaction that involves the transfer of electrons between two species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. Redox Reaction, Oxidation, and Reduction Reactions with exact equations are common and vital to some of the basic functions of life, including photosynthesis, respiration, combustion, and corrosion or rusting.
- Oxidation: The process in which there is loss of electrons.
- Reduction: The process in which there is a gain of electrons.
- Oxidizing Agent: A substance that brings about oxidation.
- Reducing Agents: A substance that brings about reduction.
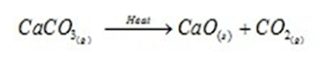
viii). Endothermic Reactions: Reaction in which heat is absorbed to carry out the chemical reaction. For Example:
- CaCO3 (s) + Heat → CaO (s) + CO2 (g) Calcium Calcium Carbon
Carbonate Oxide Dioxide
- C(s) + H2 O (s) + Heat → CO (g) + H2 (g)
Carbon Carbon
Monoxide (Evolve)
ix). Exothermic Reactions: Reaction in which heat is evolved during the chemical reaction. For Example:
- C + O2 → CO2 (g) + heat
Chemical Reactions And Equations Class 10 Notes
The Effect of Oxidation-Reduction in Daily Life Corrosion
Many metals are chemically active elements and get easily affected by substances like moisture, air, acids, etc, and undergo Oxidation, Reduction in Daily Life.
- Corrosion – The process of slow conversion of metals into their undesirable compounds due to their reaction with oxygen, water, acids, gases, etc. present in the atmosphere is called corrosion.
- Rusting of Iron: We know iron articles that are shiny when new and get coated with a reddish-brown powder when left for some time this process is called Rusting of Iron. Chemically, Rust is a hydrated ferric oxide (Fe2O3.xH2O)
- Advantages of Corrosion: Though corrosion is undesirable, it can be advantageous in the case of aluminum which on exposure to air, gets coated with a protective layer of aluminum oxide. This protects the metal underneath from further corrosion and damage.
- The black coating on silver and the green coating on copper are examples of corrosion in which the oxides formed a strong bond to the surface of the metal, preventing the surface from further exposure to oxygen and consequently slowing down corrosion.
- Rusting of iron can be prevented by painting, oiling the surface, or by galvanization.
- Rancidity: When oils and fats or foods containing oils and fats are exposed to air, they get oxidized due to which the food becomes stale (no longer fresh and pleasant to eat; hard or dry) and gives a bad taste or smell this is called Rancidity. The most important cause of rancidity is the deterioration in fats and fatty foods because of the oxidation process. When an oxygen atom replaces a hydrogen atom in the fatty acid molecule it destabilizes the molecule. Factors that accelerate fat oxidation include salt, light, water, bacteria, molds trace metals (iron, zinc, etc.). Above mentioned topics are important examples of Oxidation-Reduction in Daily Life
It can be prevented by using various methods such as by adding antioxidants to the food materials, Storing food in an airtight container, and flushing out air with nitrogen.
What you have learned
- A complete chemical equation represents the reactants, products, and their physical states symbolically.
- A chemical equation is balanced so that the numbers of atoms of each type involved in a chemical reaction are the same on the reactant and product sides of the equation. Equations must always be balanced.
- In a combination reaction, two or more substances combine to form a new single substance.
- Decomposition reactions are opposite to combination reactions. In a decomposition reaction, a single substance decomposes to give two or more substances.
- Reactions in which heat is given out along with the products are called exothermic reactions.
- Reactions in which energy is absorbed are known as endothermic reactions. When an element displaces another element from its compound, a displacement reaction occurs.
- Two different atoms or groups of atoms (ions) are exchanged in double displacement reactions.
- Precipitation reactions produce insoluble salts.
Oxidation-Reduction in Daily Life also involves the gain or loss of oxygen or hydrogen by substances. Oxidation is the gain of oxygen or loss of hydrogen. The reduction is the loss of oxygen or the gain of hydrogen.
Chemical Reactions And Equations Class 10 Notes