Atomic Properties with Pictorial Representation
Atomic Properties with Pictorial Representation:
Atomic Properties: The properties such as atomic radius, ionic radius, ionisation energy, electronegativity, electron affinity, and valence, called atomic properties.
Atomic Radius: It is defined as the distance from the centre of the nucleus to the outermost shell containing electrons. Atomic radius is commonly expressed in picometre (pm) or angstrom (Å).it is measured by x-ray diffraction method or by spectroscopic methods.

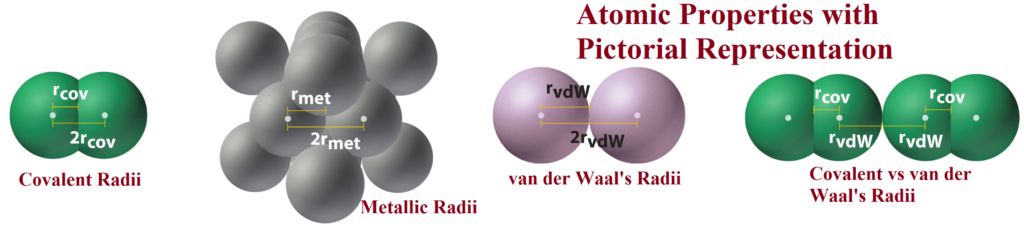
Covalent radius: One-half of the distance between the nuclei of two covalently bonded atoms of the same element in a molecule (used for non-metals).

Van der Waals radius: One half of the distance between nuclei of two identical atoms of separate molecules called van der Waals radii.

Metallic radius: One-half of the internuclear distance between the two adjacent metal ions in the metallic lattice.

Van der waal’s radius > metallic radius > covalent radius
Atomic Properties with Pictorial Representation
Variation of Atomic Radii Along the Period and Down the Group: The atomic size decreases from left to right in a period. This is because, in a period, the electrons are added to the same valence shell. Thus, the number of shells remains the same, but the effective nuclear charge increases. So, the atomic radius decreases. In a given period, alkali metals (group 1) have the maximum size and halogens (group 17) have the minimum size.
Down a group, the atomic radius increases from top to bottom. This is because of the increase in no. of shells and the Shielding Effect (in atoms with higher atomic numbers, the inner electrons partially shield the attractive force of the nucleus. So the outer electrons do not experience the full attraction of the nucleus and this is known as the shielding effect or screening effect).
Atomic radius of noble gases is larger than that of halogens. This is because noble gases are monoatomic. So van der Waal’s radius is used to express the atomic radius which is greater than covalent radius or metallic radius.
Ionic Radius: Effective distance from the centre of the nucleus of the ion up to which it exerts its, influence on its electronic cloud.
Generally, a cation is smaller than its parent atom (e.g. Na+ is smaller than Na atom). This is because a cation has fewer electrons, but its nuclear charge remains the same as that of the parent atom. An anion is larger than its parent atom (e.g. Cl– is larger than Cl atom). This is because the addition of one or more electrons would result in an increased electronic repulsion and a decrease in effective nuclear charge.

The size of inert gases is larger than halogens because in inert gases all orbitals are completely filled, inter electronic repulsions are maximum.
Isoelectronic Species: The species having the same number of electrons in atomic and ionic state are called isoelectronic species. E.g. O2-, F–, Ne, Na+, Mg2+ (All these contain 10 electrons)
Variation of ionic radii among isoelectronic species Al3+ < Mg2+ < Na+ < F–< O2- < N3-. As the effective nuclear charge increases, size decreases.

Atomic Properties with Pictorial Representation