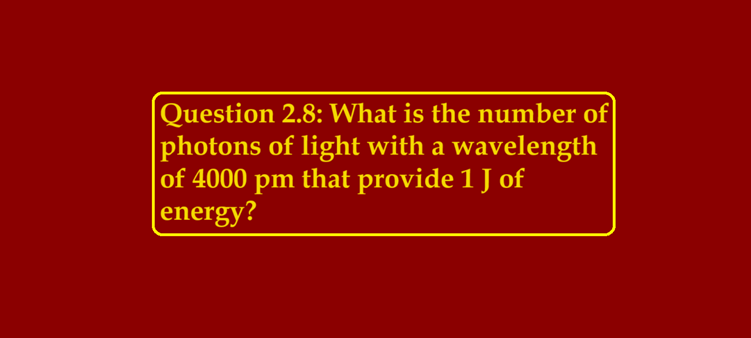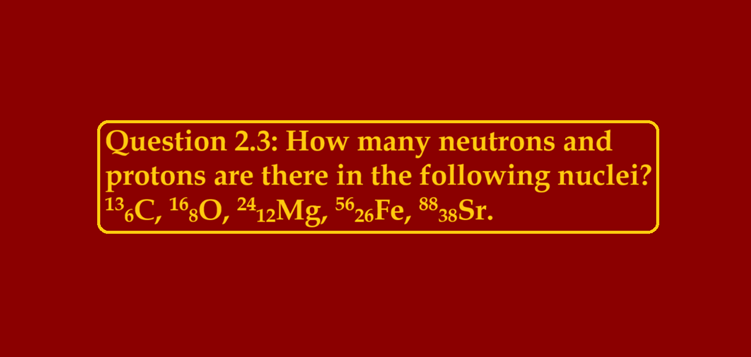Atoms and Molecules Class 9 Questions (MCQs) Atoms and Molecules Class 9 Questions Que 1. Which of the following correctly represents 360 g of water? (i) 2 moles of water (ii) 20 moles of water (iii) 6.022 × 1023 molecules of water (iv) 1.2044 × 1025 molecules of water (a) (i) (b) (i) and (iv) (c) (ii) and (iii) (d) (ii) and (iv) Que 2. Which of the following statements is not true about an atom? (a) Atoms are not able to exist independently. (b) Atoms are the basic units from which molecules and ions are formed. (c) Atoms are…
Author: Dr. Vikas Jasrotia
Question 2.12: Electrons are emitted with zero velocity from a metal surface when it is exposed to radiation of wavelength 6800 Å. Calculate the threshold frequency (ν0) and work function (W0) of the metal. https://www.youtube.com/watch?v=g26ITvZzxEI&t=10s Question 2.12: Electrons are emitted with zero velocity from a metal surface when it is exposed to radiation of wavelength 6800 Å. Calculate the threshold frequency (ν0) and work function (W0) of the metal. Answer 2.12: Threshold wavelength of radiation (Λ0) = 6800 Å = 6800 × 10–10 m = 6.8 × 10–7m Threshold frequency (ν0) of the metal ν0 = c/Λ0 c = 3…
Question 2.11: A 25-watt bulb emits monochromatic yellow light of wavelength of 0.57µm. Calculate the rate of emission of quanta per second. https://www.youtube.com/watch?v=i1KVhxl01OU&t=152s Question 2.11: A 25-watt bulb emits monochromatic yellow light of wavelength of 0.57µm. Calculate the rate of emission of quanta per second. Answer 2.11: Power of bulb, P = 25 Watt = 25 Js–1 The energy of one photon, E = hν = hc/Λ Substituting the values of h, c, and Λ in the above relation E = (6.626 × 10-34) (3 × 108)/0.57 × 10-6 E = 34.87 × 10–20 J Rate of emission of quanta…
Question 2.10: Electromagnetic radiation of wavelength 242 nm is just sufficient to ionize the sodium atom. Calculate the ionization energy of sodium in kJ mol–1. https://www.youtube.com/watch?v=fEErWcYZYYE Question 2.10: Electromagnetic radiation of wavelength 242 nm is just sufficient to ionize the sodium atom. Calculate the ionization energy of sodium in kJ mol–1. Ans 2.10: The energy of sodium is E = Nhc/Λ Given Λ = 242 nm = 242 × 10-9 m Avogadro’s No (N) = 6.023 × 1023 Planks Constant (h) = 6.626 × 10-34 Js Velocity of light (c) = 3 × 108 m/s = 6.023 × 1023 × 6.626…
Question 2.9: A photon of wavelength 4 × 10–7 m strikes on the metal surface, the work function of the metal is 2.13 eV. Calculate (i) the energy of the photon (eV), (ii) the kinetic energy of the emission, and (iii) the velocity of the photoelectron (1 eV= 1.6020 × 10–19 J). https://www.youtube.com/watch?v=RFJcrhd0ius Question 2.9: A photon of wavelength 4 × 10–7 m strikes on the metal surface, the work function of the metal is 2.13 eV. Calculate (i) the energy of the photon (eV), (ii) the kinetic energy of the emission, and (iii) the velocity of the photoelectron…
Question 2.8: What is the number of photons of light with a wavelength of 4000 pm that provide 1 J of energy? https://www.youtube.com/watch?v=lCdbg6Dtw3I Question 2.8: What is the number of photons of light with a wavelength of 4000 pm that provide 1 J of energy? Ans 2.8: Energy (E) of a photon = hν Energy (En) of ‘n’ photons = nhν ν = c/Λ n = EnΛ /hc Where, λ = wavelength of light = 4000 pm = 4000 ×10–12 m c = velocity of light in vacuum = 3 × 108 m/s h = Planck’s constant = 6.626 ×…
Question 2.7: Calculate the wavelength frequency and wave number of a light wave whose period is 2.0 × 10–10 s. https://www.youtube.com/watch?v=G4rzkF41-58 Answer 2.7: Frequency (ν) of light = 1/Period Frequency (ν) = 1/2.0 × 10–10 s = 5 × 109 s-1 Wavelength (λ) of light = c/ ν c = velocity of light in vacuum = 3×108 m/s Substituting the value in λ = c/ ν = 3 × 108 ms-1/5 × 109 s-1 λ = 6 × 10-2 m Wave number of light = 1/wavelength = 1/6 × 10-2 m = 16.66 m-1
Class 11 Chemistry Chapter 2 Question 2.1 https://www.youtube.com/watch?v=_lNTTItFsbg&t=32s Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Ans 2.1: (i) Mass of one electron = 9.1 × 10–31 kg Number of electrons that weigh 9.1 × 10–31 kg = 1 Number of electrons that will weigh 1 g i.e 1 × 10–3 kg = 1 × 10–3 kg/ 9.1 × 10–31 kg = 0.1098 × 10–3 + 31 = 0.1098 × 1028 = 1.098 × 1027 (ii) Mass of one electron = 9.1 ×…
https://www.youtube.com/watch?v=wPSp-xSYWsw Question 2.4: Write the complete symbol for the atom with the given atomic number (Z) and atomic mass (A) (i) Z = 17, A = 35 (ii) Z = 92, A = 233 (iii) Z = 4, A = 9 Ans 2.4: For any given atom, we can write the symbols as AZX. Here, X is the element’s symbol, Z is its atomic number and A is its mass number. Therefore the symbols for the above elements are written as: (i) Z = 17, A = 35 Symbol: 3517Cl (ii) Z = 92, A = 233 Symbol: 23392U (iii)…
https://www.youtube.com/watch?v=6-di0ujG_Ew Question 2.3: How many neutrons and protons are there in the following nuclei? 136C, 168O, 2412Mg, 5626Fe, 8838Sr Ans 2.3: 136C Atomic mass = 13 Atomic number = Number of protons = 6 Number of neutrons = Atomic Mass – Atomic Number ∴ Number of neutrons = 13 – 6 = 7 168O Atomic mass = 16 Atomic number = 8 Number of protons = 8 Number of neutrons = Atomic Mass – Atomic Number ∴ Number of neutrons = 16 – 8 = 8 2412Mg Atomic mass = 24 Atomic number = Number of protons = 12…