Electromeric Effect
An Electromeric effect is a temporary effect that takes place between two atoms joined by multiple bonds for means a double or triple bond. When the charged reagents like electrophiles or nucleophiles approach them, the electrons get polarized and displaced towards one of the constituents of the atom. The electromeric effect is a reversible reaction where there is a complete transfer of a pi-electron pair due to the influence of an electrophile or a nucleophile. The effect disappears upon withdrawal of the attacking reagent.
Electromeric Effect
Types of Electromeric Effects
The electromeric effect can be broken down into two types. This classification is done based on the direction in which the electron pair is transferred.
(i) The +E effect, which is also referred to as the positive electromeric effect
(ii) The -E effect, which is also referred to as the negative electromeric effect
(i) The +E effect: This effect occurs when the electron pair of the pi bond is moved towards the attacking reagent. The +E effect can be observed in the addition of acid to alkenes.
The +E effect is generally observed when the attacking reagent is an electrophile and the pi electrons are transferred towards the positively charged atom.
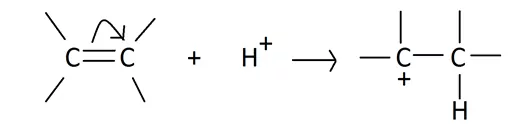
(ii) The -E effect: This effect occurs when the electron pair of the pi bond is moved away from the attacking reagent. The -E effect is observed in the reaction of the addition of cyanide ions to carbonyl compounds. When a negatively charged reagent approaches a molecule seeking partially positive carbon, it causes an instantaneous shift of an electron pair of the C=O group towards the more electronegative oxygen atom. Hence, carbon becomes deprived of its share in this transferred pair of electrons and acquires a positive charge.
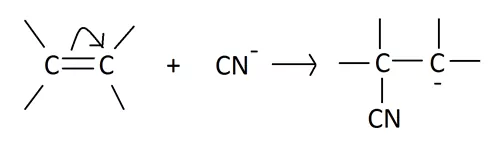
Now let us check the characteristics of the electromeric effect from our discussion. A few of them are:
(i) It is a temporary effect that involves a complete transfer of π-electrons to one of the bonded atoms.
(ii) It operates in unsaturated compounds which contain at least one multiple bonds which may be polar or non-polar.
(iii) It operates only in the presence of an attacking reagent.
(iv) It involves a complete transfer of electrons from the reagent to the substrate and vice versa. As a result of this effect, ions are formed.



