Sulphur its Compounds and Properties
Sulphur (S): Sulphur is the 2nd element of the oxygen family. Sulphur forms a large number of allotropes. Among these Yellow Rhombic (α-Sulphur) and Monoclinic (β -Sulphur) forms are the most important. The stable form at room temperature is rhombic sulphur, which transforms into monoclinic sulphur when heated above 369 K.
(i) Rhombic Sulphur (a – Sulphur): This allotrope is yellow in colour, m.p. 385.8 K and specific gravity 2.06. Rhombic sulphur crystals are formed on evaporating the solution of roll sulphur in CS2. It is insoluble in water but dissolved to some extent in benzene, alcohol and ether. It is readily soluble in CS2.
(ii) Monoclinic (β -Sulphur): It is prepared by melting rhombic sulphur in a dish and cooling, till a crust is formed. Two holes are made in the crust and the remaining liquid is poured out. On removing the crust, colourless needle-shaped crystals of β-sulphur are formed. It is stable above 369 K and transforms into α-sulphur below it. At 369 K both the forms are stable. This temperature is called transition temperature.
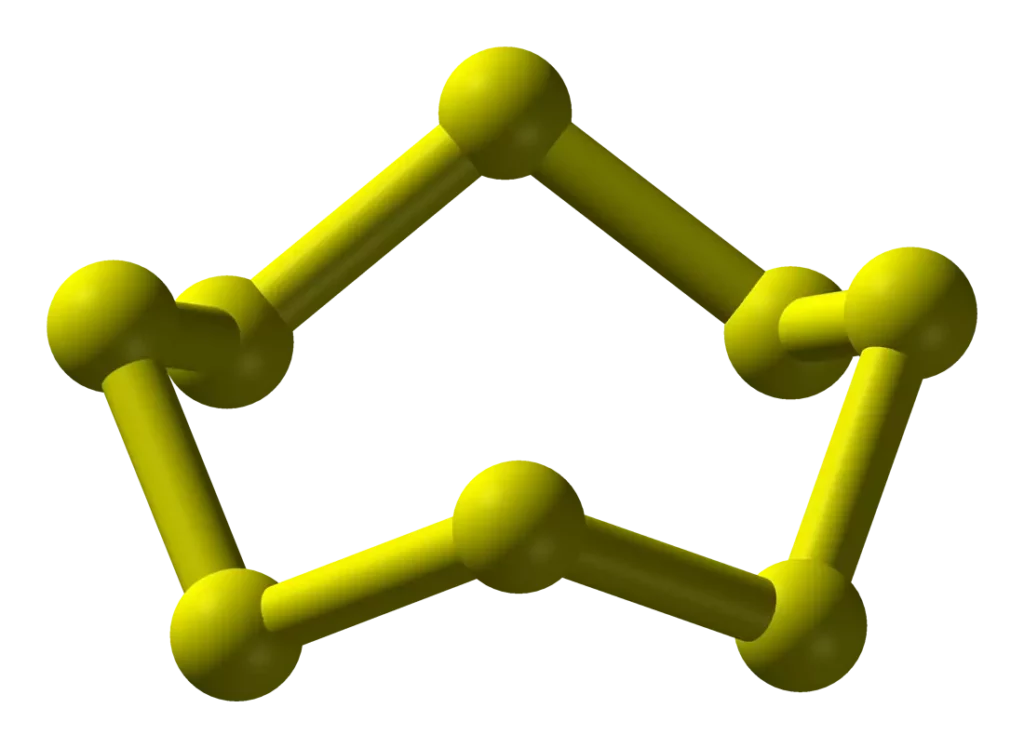
Both rhombic and monoclinic sulphur have S8 molecules. The S8 ring in both forms is puckered and has a crown shape.
Sulphur its Compounds and Properties
Sulphur Dioxide (SO2):
(i) Preparation:
Sulphur dioxide is formed when sulphur is burnt in air or oxygen: S(s) + O2(g) → SO2 (g)
In the laboratory, it is obtained by treating a sulphite with dilute sulphuric acid.
SO32-(aq) + 2H+ (aq) → H2O(l) + SO2 (g)
Industrially, it is produced by roasting of sulphide ores.
4 FeS2(s) + 11 O2(g) → 2 Fe2O3(s) + 8 SO2(g)
(ii) Properties:
- Sulphur dioxide is a colourless gas with a pungent smell and is highly soluble in water.
- With water, it forms a solution of sulphurous acid which is a dibasic acid and forms two types of salts with alkalies – normal salt (sulphite) and acid salt (bisulphate or hydrogen sulphite).
SO2(g) + H2O(l) → H2SO3(aq) - Neither burns nor helps in burning but burning magnesium and potassium continue to burn in its atmosphere.
3Mg + SO2 → 2 MgO + MgS
4K + 3SO2 → K2SO3 + K2S2O3
- With sodium hydroxide solution, it forms sodium sulphite, which then reacts with more sulphur dioxide to form sodium hydrogen sulphite.
2NaOH + SO2 → Na2SO3 + H2O
Na2SO3 + H2O + SO2 → 2NaHSO3
- SO2is oxidised to sulphur trioxide by oxygen in the presence of vanadium pentoxide (V2O5) catalyst. 2SO2 + O2 → 2SO3
- Moist sulphur dioxide behaves as a reducing agent. It converts iron (III) ions to iron (II) ions and decolourises acidified potassium permanganate(VII) solution (This used as a test for SO2).
2Fe3+ + SO2 + 2H2O → 2Fe2+ + SO42- + 4H+
5 SO2 + 2MnO4– + 2H2O → 5 SO42- + 4H+ + 2Mn2+
Acidified K2Cr2O7 → Cr3+ (green coloured solution)
Bleaching Action: SO2 + 2H2O → H2SO4 + 2H
This is due to the reducing nature of SO2
Coloured matter + H ↔ Colourless matter. Therefore, the bleaching is temporary.
Uses: Sulphur dioxide is used:
- Used in the manufacture of H2SO4& paper from wood pulp.
- As a bleaching agent for delicate articles like wool, silk and straw.
- Used in the refining of petroleum and sugar.
- Liquid SO2is used as a solvent to dissolve a number of organic and inorganic chemicals.
Sulphur its Compounds and Properties
Oxoacids of Sulphur: Sulphur forms a large no. of oxoacids. Sulphurous acid (H2SO3), Sulphuric acid (H2SO4), Dithionic acid (H2S2O6), Peroxomonosulphuric acid (Caro’s acid, H2SO5), Peroxodisulphuric acid (Marshell’s acid, H2S2O8) etc. The structure of some oxoacids are
Sulphur its Compounds and Properties
Sulphuric acid (H2SO4): One of the most important oxoacids of sulphur is H2SO4. It is also known as the king of all acid.
- Manufacture: Sulphuric acid is manufactured by the Contact Process which involves three steps:
- Manufacture: Sulphuric acid is manufactured by the Contact Process which involves three steps:
Step 1: Burning of sulphur or sulphide ores in the air to generate SO2.
S(s) + O2(g) → SO2 (g)
OR
4FeS2(s) + 11 O2(g) → 2 Fe2O3(s) + 8 SO2(g)
Step 2: Conversion of SO2 to SO3 by the reaction with oxygen in the presence of a catalyst (V2O5).
2SO2 + O2 → 2SO3
Step 3: Absorption of SO3 in H2SO4 to give Oleum (H2S2O7).
SO3 + H2SO4 → H2S2O7
Dilution of oleum with water gives H2SO4 of the desired concentration.
H2S2O7 + H2O → 2H2SO4.
Lead Chamber Process (Industrial method):
2SO2 + O2 (air) + 2H2O + [NO] (catalyst) ¾® 2H2SO4 + [NO] (catalyst)
Acid obtained is 80% pure and is known as Brown Oil of Vitriol.
Properties of Sulphuric acid:
- Sulphuric acid is a colourless, dense, oily liquid.
- It dissolves in water with the evolution of a large quantity of heat. Hence, for diluting the acid, the concentrated acid must be added slowly into the water with constant stirring.
- It fumes strongly in moist air and is highly corrosive in nature.
- Thermal decomposition: It decomposes at 440 0C
H2SO4 ↔H2O+SO3
- The chemical reactions of sulphuric acid are due to
(i) Its low volatility
(ii) Strong acidic character
(iii) Strong affinity for water and
(iv) Its ability to act as an oxidising agent.
In aqueous solution, sulphuric acid ionises in two steps.
H2SO4(aq) + H2O(l) → H3O+(aq) + HSO4–
HSO4–(aq) + H2O(l) → H3O+(aq) + SO42-
So, it is dibasic and forms two series of salts: Normal Sulphates and Acid
Sulphates.
- Decomposes carbonates and bicarbonates into carbon dioxide
Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2
NaHCO3 + H2SO4 → NaHSO4 + H2O + CO2
Concentrated sulphuric acid is a strong dehydrating agent and drying agent. Many wet gases can be dried by passing them through sulphuric acid. Sulphuric acid removes water from organic compounds
e.g.: C12H22O11 + H2SO4 → 12C + 11H2O
- Hot concentrated sulphuric acid is a moderately strong oxidising agent. It oxidises both metals and non-metals and the acid itself reduces to SO2.
Cu + 2 H2SO4(conc.) → CuSO4 + SO 2 + 2H2O
S + 2H 2SO4(conc.) → 3SO2 + 2H2O
C + 2H2SO4(conc.) → CO2 + 2 SO2 + 2 H2O
Uses: The important uses of Sulphuric acid are:
- In the manufacture of fertilizers
- In petroleum refining
- In the manufacture of pigments, paints and dyestuff intermediates.
- In the detergent industry
- In metallurgical applications
- As electrolyte in storage batteries.
- In the manufacture of nitrocellulose products and
- As a laboratory