Coordination Compounds Important Topics
The concept of coordination compounds originates from the tendency for the complex formation of the transition elements.
Complex compounds or coordination compounds are those molecular compounds that retain their identity in solid and in solution are known as complex compounds.
K4[Fe(CN)6 ] + H2O → 4K+(aq) + [Fe(CN)6]4− (aq)
Molecular or Addition Compounds:
When solutions of two or more simple stable compounds in molecular proportion are allowed to evaporate, crystals of new substances, called molecular or addition compounds, are obtained. For Example:
CuSO4 + 4NH3 → CuSO4·4NH3
AgCN + KCN → KCN·AgCN
There are two types of molecular or addition compounds: Double salts or lattice compounds and Coordination or complex compounds.
(1) Double salts or lattice compounds: These are the molecular compounds that exist only in the solid-state and lose their identity when dissolved in water, i.e., they dissociate into simple ions completely when dissolved in water. For Example FeSO4. (NH4)2SO4.6H2O (Mohr’s Salt), K2SO4. Al2(SO4)3.24H2O (Potash Alum).
(2) Coordination or Complex Compounds: These are the molecular compounds that retain their identity in the solid as well as in the dissolved state and their properties are completely different from those of their constituent particles. A part of these compounds is not dissociated in solution and its behavior is different from its constituents.
For example, potassium argentocyanide does not form Ag+ and CN– simpler ions but instead, gives argentocyanide complex ion [Ag(CN)2]– (which does not dissociate).
Cationic complexes: A complex in which the complexion carries a net positive charge is called a cationic complex. For Example, [CoCl2(en)2]+, [Co(NH3)6]3+, [Cu(NH3)4]2+.
Anionic complexes: A complex in which the complexion carries a net negative charge is called anionic complex, e.g., [Ag(CN)2]–, [Fe(CN)6]4-, [Fe(C2O4)3]3−.
Neutral complexes: A complex carrying no net charge is called a neutral complex or simply a complex e.g., [Ni(CO)4], [Co(NH3)3Cl3], [Ni(CO)4].
Coordination Compounds Important Topics
Some Important Definition
1) Coordination Entity: Central atom/ion (metal) to which a fixed number of other atoms or groups are attached (ligand). For example, [CoCl3(NH3)3] is a coordination entity in which the cobalt ion is surrounded by three ammonia molecules and three chloride ions. Other examples are [Ni(CO)4], [PtCl2(NH3)2], [Fe(CN)6]4–, [Co(NH3)6]3+.
A coordination entity can be neutral or charged.
2) Central atom/ion: In a coordination entity, the atom/ion to which a fixed number of ions/neutral molecules are attached is called the central atom or ion. For example, the central atom/ion in the co-ordination entities: [NiCl2(H2O)4], [CoCl(NH3)5]2+ and [Fe(CN)6]3– are Ni2+, Co3+ and Fe3+ respectively.
These central atoms/ions are also referred to as Lewis acids since they accept electron pairs from ligands.
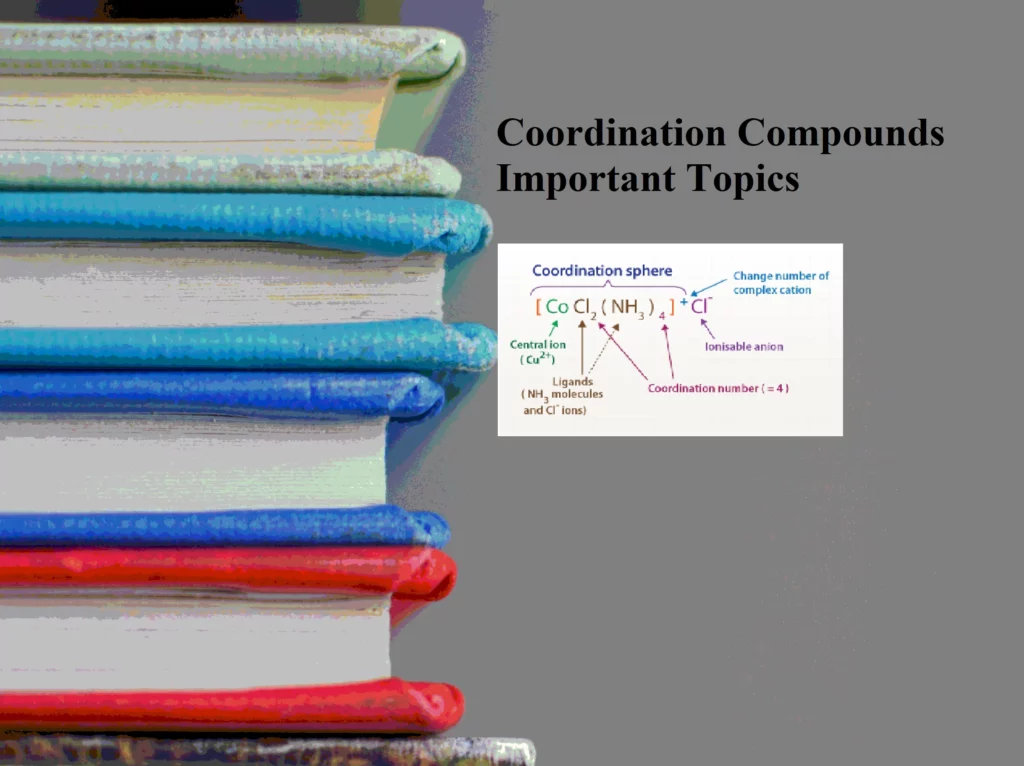
3) Ligands: The anions, cations, or neutral molecules, which form coordinate bonds with the central metal atom by donating an electron pair (lone pair) are ligands. These electron-pair donors are also known as Lewis bases. The atom in the ligand which donates the electron pair is called the donor atom or ligating group. The ligands containing one, two, or more donor atoms are known as unidentate, bidentate, or multidentate respectively. For Example, Examples for ligands are Cl−, Br−, F−, I−, OH−, CN−, NC−, CNO−, NCO−, SO42−, NO3−, CNS–, H2O, NH3, CO, etc.
Classification of Ligands:
Ligands are classified as follows:
(a) On the basis of the charge of ligand:
i) Anionic ligands: These are negatively charged and are the most common type of ligand, such as F–, Cl–, Br–, OH–, CN–, SO32–, S2–, SO42–, etc.
ii) Neutral ligands: These are uncharged and are the electron-pair donor species such as H2O, ROH, NH3, RNH2, R2NH, R3N, etc.
iii) Cationic ligands: They are positively charged and are rare such as NO+, etc.
b) On the basis of denticity: The number of donations accepted by a central atom from a particular ligand is known as the denticity of the ligand.
Based on this, ligands are classified as follows:
i) Monodentate or Unidentate Ligands: A ligand that is bound to a metal ion through a single donor atom. e.g., H2O, NH3, CO, Cl−, NH2−
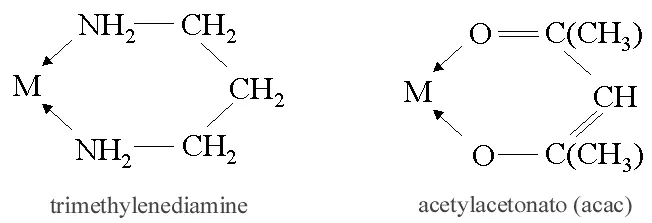
ii) Bidentate (Didentate) ligands: A ligand that binds to the central atom through two donor atoms is called a bidentate ligand. Example: Ethane-1,2-diamine or ethylenediamine (H2NCH2CH2NH2) notated as ‘en’ and oxalate ion (C2O42–) notated as ‘en’.
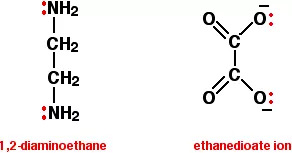
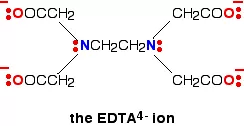
iii) Polydentate ligand: A ligand that binds to the central atom through more than two donor atoms is called a polydentate ligand. For Example Triethylamine ammonia [N(CH2-CH2-NH2)3], Ethylenediamine tetraacetate ion (EDTA4–), etc. Ethylenediamine tetraacetate ion (EDTA4–) is an important hexadentate ligand. It can bind through two nitrogen and four oxygen atoms to a central metal ion.
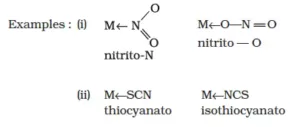
iv) Ambidentate ligands: They are unidentate ligands that contain more than one donor atom. They can coordinate through two different atoms. Examples of such ligands are the NO2–, CN–, SCN–, CNO–.
 v) Chelating ligands: A ligand that forms a ring structure with the central atom is called a chelating ligand. All polydentate ligands are chelating ligands.
v) Chelating ligands: A ligand that forms a ring structure with the central atom is called a chelating ligand. All polydentate ligands are chelating ligands.
Coordination Compounds Important Topics
4) Co-ordination number: The number of atoms in a ligand that directly bond to the central metal atom or ion by coordinate bonds is called the coordination number of the metal atom or ion. Some common coordination numbers exhibited by metal ions are 2, 4, and 6. The light transition metals exhibit 4 and 6 coordination numbers while heavy transition metals exhibit coordination numbers above 6.
For example, in the complex ion [PtCl6]2– the coordination number of Pt is 6 and in [Ni(NH3)4]2+, the coordination number of Ni is 4. Similarly, in the complexions, [Fe(C2O4)3]3– and [Co(en)3]3+, the coordination number of both Fe and Co, is 6 because C2O42– and en (ethane-1,2- diamine) are bidentate ligands.
5) Co-ordination sphere: The central metal atom/ion and the ligands directly attached to it are collectively termed as the coordination sphere. The coordination sphere is represented inside square brackets, e.g. [Fe(CN)6]4–.
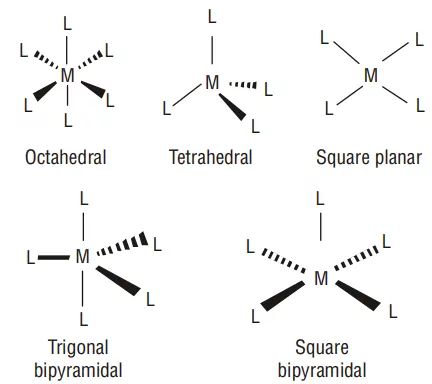
6) Co-ordination polyhedron: The spatial arrangement of the ligands around the central atom/ion defines a coordination polyhedron about the central atom. The most common coordination polyhedra are octahedral, square planar, and tetrahedral. For example, [Co(NH3)6]3+ is octahedral, [Ni(CO)4] is tetrahedral and [PtCl4]2– is square planar.
Coordination Compounds Important Topics
7) Oxidation Number: The actual charge that a metal atom experiences in a complex are known as its oxidation number. In other words, the oxidation number of a metal atom will be equal to the total charge on this atom if all the ligands are removed without their electron pair. For Example: Find the oxidation number of Co in the complex [Co (en)2 (H2O) (CN)]2+.
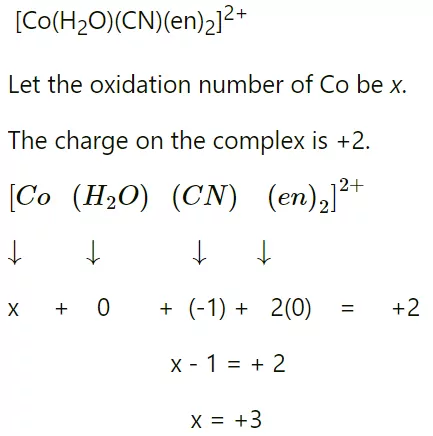
8) Homoleptic and Heteroleptic Complexes: Complexes in which the central metal atom or ion is linked to the only types of ligands are called homoleptic complexes. For example, [Co(NH3)6]3+.
The complexes in which the central metal atom or ion is linked to more than one kind of ligand are called heteroleptic complexes. For example, [Co(NH3)4Cl2]+.
Coordination Compounds Important Topics