Biomolecules Class 12 Questions and Answers
Biomolecules Class 12 Questions and Answers
Que 1. What are monosaccharides?
Ans 1. They are single carbohydrates, i.e., polyhydroxy aldehydes (aldoses) or polyhydroxy ketones (ketoses) which cannot be broken into lower sugars upon hydrolysis. They have the general formula, CnH2nOn, where n varies from 3 to 9 carbon atoms. The common monosaccharides are glucose, fructose and ribose, etc.
Que 2. What is glycosidic linkage? Give one example
Ans 2. The two monosaccharide units are joined together through an oxide (ethereal) linkage formed by the loss of a molecule of H2O. Such a linkage between two monosaccharide units through the oxygen atom of a molecule of a disaccharide is called glycosidic linkage. For example, a glycosidic linkage is present in sucrose, maltose, lactose, etc.
Que 3. Why are carbohydrates generally optically active?
Ans 3. Carbohydrates are optically active as they provide polyhydroxy aldehydes or ketones on hydrolysis. Their molecules are chiral because of the presence of many asymmetric carbon atoms.
Que 4. How will you differentiate between sugars and non-sugars?
Ans 4. Sugars: they are monosaccharides and disaccharides, sweet in taste, soluble in water and are crystalline solid.
Non-sugars: They are polysaccharides that are not sweet in taste, insoluble in cold water
Que 5. What is meant by reducing sugars?
Ans 5. Reducing sugars reduces Tollen’s reagent to metallic silver or Fehling’s solutions to red ppt. Of Cu2O or Benedict’s solutions. Examples are glucose, mannose, galactose, fructose, etc. (all monosaccharides) and also disaccharides such as maltose, lactose, etc. (except sucrose) are reducing sugars.
Biomolecules Class 12 Questions and Answers
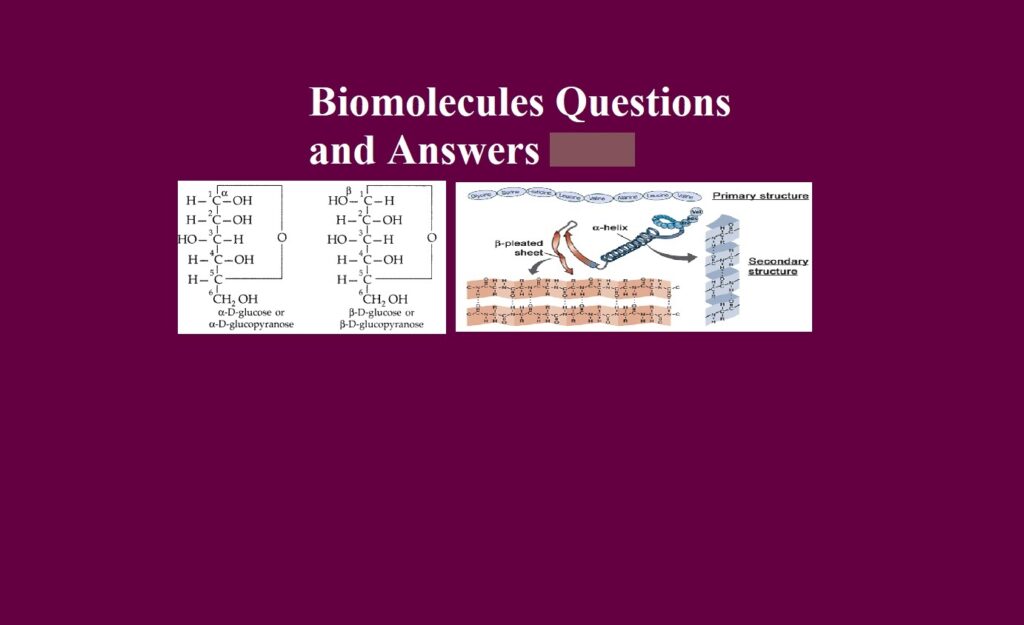
Que 6. What is an anomeric carbon atom?
Ans 6. The first asymmetric carbon atom in cyclic compounds on which the configurations of H – and OH – groups are different is called an anomeric C-atom. For example, α-glucose and β-glucose (they are anomers).
Que 7. What is mutarotation?
Ans 7. The process which involves the change in optical rotation of either form of glucose (or fructose) in aqueous solutions to that of equilibrium mixture is called mutarotation.
Que 8. What is meant by the pyranose structure of glucose?
Ans 8. The six-membered cyclic structure of glucose (i.e., the ring is formed between C1 and C5) is called pyranose structure in analogy with pyran heterocyclic compound or structure.
Que 9. Give the types of classification of proteins on the basis of shape (molecular structure with certain examples?
Ans 9. (i) Fibrous protein: Examples are, Keratin, Myosin. Fibroin, Collagen. Etc.
(ii) Globular proteins: Examples are, Haemoglobin, Insulin, Enzymes, Antibodies, etc.
Que 10. What types of bonding occurs in globular proteins?
Ans 10. Intramolecular H-bonding, disulphide bridge, dipolar interactions (ionic) hydrophobic interactions.
Biomolecules Class 12 Questions and Answers
Que 11. Name of the following:
(i) A water-soluble vitamin
(ii) An oil-soluble vitamin
(iii) A vitamin that is neither soluble in water nor is fat
(iv) The disease is caused by a deficiency of vitamin B1.
(v) The diseases caused by deficiency of vitamin C.
(vi) What do you understand by avitaminosis?
Ans 11. (i) Vitamin C
(ii) Vitamin D
(iii) Vitamin H
(iv) Beriberi
(v) Scurvy.
(vi) Avitaminosis is the condition caused by lack of more than one vitamin in the human beings
Que 12. What is the tertiary structure of proteins?
Ans 12. Further folding, twisting and bending of the secondary structure of a protein in the three-dimensional space to give a different specific compact shape is called the tertiary structure of proteins.
Que 13. What is the nucleoside?
Ans 13. A nucleoside is a combination of base and sugar. Nucleosides are obtained when a nitrogenous base (purine or pyrimidine) is attached to C1 of a sugar (ribose or deoxyribose) by a linkage.
Que 14. What are essential and non-essential amino acids? Give two examples of each.
Ans 14. The amino acids which cannot be synthesized in our body but must be supplied through our diet are known as essential amino acids. There are about 10 essential amino acids. For example, valine, leucine and lysine. The amino acids which can be synthesized in the human body are known as non-essential amino acids. There are about 10 non-essential amino acids. For example, glycine, alanine, serine.
Que 15. Explain the following terms:
(i) Native protein
(ii) Denaturation
(iii) Renaturation.
Ans 15. (i) Native protein is the protein found in a biological system with a definite configuration and biological activity.
(ii) When a protein is subjected to some physical or chemical treatment that disrupts its higher structures without affecting its primary structure, the process is called denaturation. The denatured protein loses its biological activity. For example, boiling an egg. The denaturation may be reversible or irreversible.
(iii) Renaturation. In many cases, a denatured protein recovers its physical and chemical properties and biological activity when the disruptive agent is removed. This process, which is the reverse of denaturation, is known as renaturation.
Biomolecules Class 12 Questions and Answers
Que 16. How will you distinguish between?
(a) Glucose and fructose
(b) Glucose and sucrose
(c) Glucose and starch.
Ans 16. (a) Glucose decolourise the red colour of bromine water. It is oxidised to gluconic acid. Fructose does not react with bromine water. Fructose gives a red colour with resorcinol and conc. HCl while glucose does not give any colour.
(b) Glucose on heating with Tollen’s reagent gives silver mirror while sucrose does not. Glucose gives red ppt. With Fehling’s solutions while sucrose does not.
(c) Glucose’s give positive tests with Tollen’s reagent and Fehling’s solutions while starch does not. Starch give blue colour with iodine solutions while glucose does not.
Que 17. What are peptides? How are they classified? What is a peptide bond?
Ans 17. Peptides are the products formed by the condensation of two or more amino acids through their amino and carboxylic groups involving the elimination of water molecules. They may be classified as dipeptides, tripeptides and polypeptides, depending upon whether the number of amino acid molecules taking part in condensation is two, three or more than three respectively. The linkage (-CN-NH-) which unites various amino acid units in a peptide molecule is called peptide linkage or peptide bond.
Que 18. Give reasons for the following:
(i) On electrolysis in acidic solution, amino acids migrate towards cathode while in alkaline solution these migrate towards anode.
(ii) The monoamino, monocarboxylic acids have two pKa values.
Ans 18. (a) In an acidic solution, the carboxylate anion accepts a proton and gets converted into a carboxylic group resulting in the formation of a positive ion.
In presence of a base -the NH3+ ion changes to −NH2 group by losing a proton and this gives a negative ion.
This means that in an acidic medium, the amino acid migrates towards the cathode while in an alkaline solution, it migrates towards the anode on electrolysis.
(b) In an aqueous solution, monoamine, monocarboxylic acid behave like salt at the isoelectric point. At a pH lower than the isoelectric point (i.e. in acidic medium) it shows one pKa value and at a pH higher than the isoelectric point. (i.e. basic medium), it shows another pKa value.
Biomolecules Class 12 Questions and Answers